Welcome to our Aging Stress Response Research Project Team homepage
Aging is closely related to abnormalities in adaptation to various environmental stresses such as DNA damage response, redox response to oxidative stress, inflammatory stress response to tissue damage, endoplasmic reticulum stress response, mitochondrial stress response, etc. In order to understand the aging mechanism, it is extremely important to elucidate changes of these various response mechanisms during aging process. It is also significant in aging research to understand how these aging stress response mechanisms protect or damage cells and tissues at the molecular level. The research project team especially focuses on mitochondria, which are the core organelles of energy metabolism and reactive oxygen species, and aims to elucidate the mechanism of mitochondrial stress response based on mitochondrial dysfunction. We are also studying the molecular mechanism that leads to signal transmission from mitochondria to the nucleus and epigenetics regulation during aging and age-related diseases.
Information
We have launched a special issue related to aging in the open-access journal of the International Journal of Molecular Sciences (IF = 6.208). Call for original papers and reviews. We look forward to your contribution
Special Issue "Molecular Research of Aging Stress Response
News
- October 11, 2023, Collaborative research by Dr. Naotaka Izuo of University of Toyama and Dr. Takahiko Shimizu of our laboratory has demonstrated in animal experiments that insulin resistance causes early onset of memory impairment in Alzheimer's disease, independent of persistent hyperglycemia. This research was published in the "Aging Cell". This research was also press released on October 16, 2023.NEW!
Press release of Toyama University. 
Press release of National Center for Geriatrics and Gerontology.
- July 13-15, 2023, Dr. Shibuya gave an oral presentation at the Japan Society for Biomedical Gerontology, Korea-Japan joint symposium in Seoul. “Elucidation of the onset mechanism of premature aging using novel Werner’s syndrome model mice”NEW!
- June 12-14, 2023, Dr. Shimizu, Dr. Shibuya, and Dr. Watanabe gave a poster presentation at the IAGG Asia/Oceania Regional Congress 2023 in Yokohama.NEW!
- November 28, 2022, A paper written by Dr. Shibuya was published in the online scientific journal "Biomedicines"
“Natural Compounds That Enhance Motor Function in a Mouse Model of Muscle Fatigue ”NEW!
”NEW!
- February 2, 2023, Members pages were updated
- April 19, 2021, Publications and Members pages were updated
- January 12, 2021, Publications were updated
- October 26, 2020, Publications were updated
- September 10, 2020, Publications were updated
- July 15, 2020, Publications and Members pages were updated
- May 28, 2020, Dr. Shibuya has published in the paper at the 43rd Japan Society for Biomedical Gerontology (Nagasaki)
- May 17, 2020, The English version of our website has been opened
先頭へ戻る
Our Research
1. Mitochondrial stress response research focusing on SOD2
Focusing on SOD2 (Mn-SOD), which plays a central role in redox regulation in mitochondria, we have investigated the role of mitochondrial function in organ damage processes. We have originally created organ-specific Sod2-deficient mice using a cre-loxp system and have revealed that SOD2 loss causes mitochondrial dysfunction and organ damage in each organ (Fig. 1). In particular, in skeletal muscles of locomotor organ tissues, a decrease in motor capacity due to ATP depletion was observed, bone loss due to disruption of the osteocyte network in bone tissue (Fig. 2), and cartilage degeneration due to disruption of extracellular matrix balance in cartilage tissue was also observed (Fig. 3). These pathologies closely resemble the age-related changes seen in old age, and SOD2 is markedly down-regulated in the cartilage tissue of patients with osteoarthritis, which suggests that it may reproduce the aging process of organs. Moreover, SOD2 loss leads to oxidative deactivation of a key enzyme in the metabolic network and deregulation of mitochondrial stress response, leading to some molecular mechanisms causing organ damage.
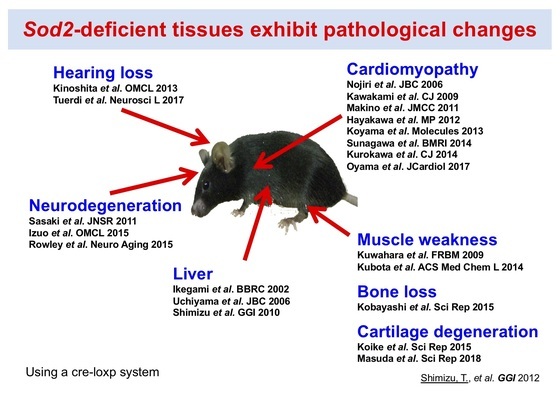
Figure 1. Sod2-deficient tissues exhibit pathological changes in various organs.
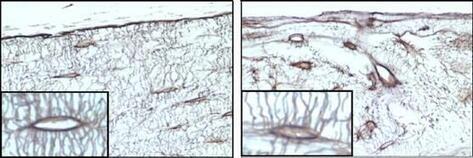
Figure 2. Femoral histology of osteocyte-specific SOD2-deficient mice. Control bone Section image of control bone (left, 5 months of age) and mutant bone (right, 5 months of age). The irregularity of the canalicular structure is noticeable.
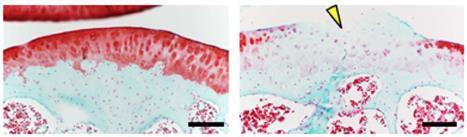
Figure 3. Knee cartilage histology of chondrocyte-specific SOD2-deficient mice. Section image of control cartilage (left, 12 months of age) and mutant cartilage (right, 12 months of age). Marked cartilage degeneration and crack are observed.
2. Redox stress response research focusing on SOD1
Focusing on SOD1 (CuZn-SOD), which plays a central role in redox regulation in the cytoplasm, we have investigated the pathophysiological role of cytoplasmic redox regulation in organ damage processes. Analyses of Sod1-deficient mice revealed that age-related changes were observed in various organs throughout the body with aging (Figs. 4 and 5). Interestingly, when we examined the level of SOD1 protein in the autopsy brain of Alzheimer's disease (AD) patients, we found that SOD1 was selectively reduced. When Sod1-deficient mice were crossed with AD model mice, we found that senile plaque formation was promoted (Fig. 6) and amyloid β (Aβ) oligomers were increased with the insufficiency in SOD1 allele, as well as memory and learning impairment. These results suggest that redox dysregulation in the brain due to decreased SOD1 may contribute to the development of Alzheimer's disease. Recently, an increase in cell senescence markers has also been observed in Sod1-deficient tissues, and it has been attracting attention as a new model mouse for promoting aging.
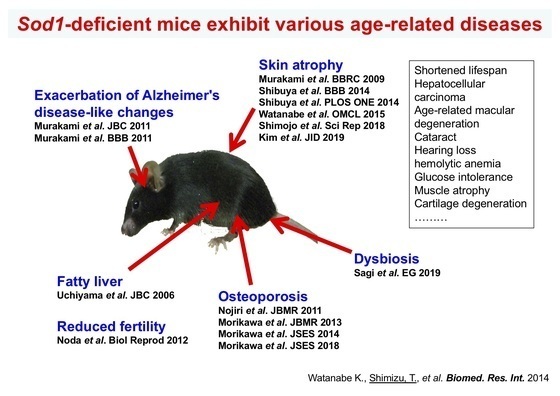
Figure 4. Sod1-deficient mice exhibit various age-related diseases.
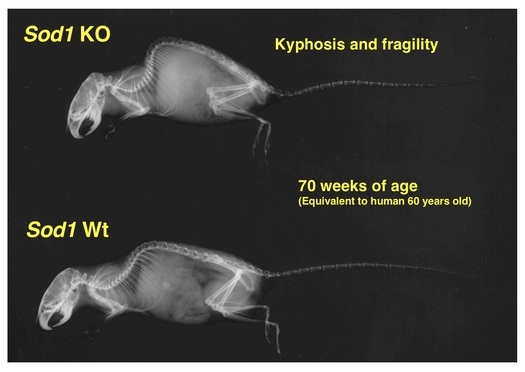
Figure 5. Sod1-deficient mice exhibit osteoporosis with kyphosis.
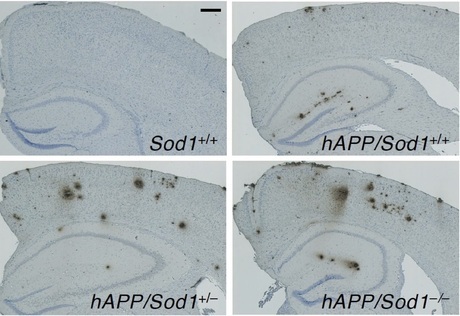
Figure 6. AD model brain with SOD1 insufficiency promotes senile plaque formation.
3. Research on organ aging control by functional foods
Since our aging model mice exhibit premature aging, it is possible to investigate the protective effect of drugs and food factors against organ aging in vivo. Furthermore, by investigating the effects of organ damage caused by oxidative stress, we can clarify the protective mechanism of substances. A Ginseng component, syringaresinol, normalized skin atrophy in Sod1-deficient mice by regulating the FoxO3a-MMP-2 axis (Fig. 7). In addition, apple procyanidin mitigated cartilage degeneration by its mitochondrial biogenesis and proteoglycan production activities (Fig. 8). We are also conducting research to clarify the new value of functional food factors.
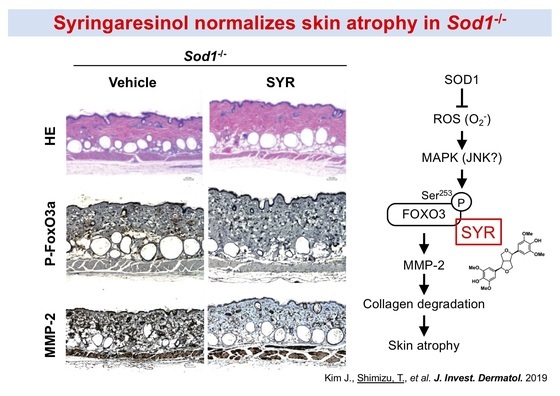
Figure 7. A Ginseng component, syringaresinol, normalizes skin atrophy in Sod1-deficient mice by regulating FoxO3a-MMP-2 axis.
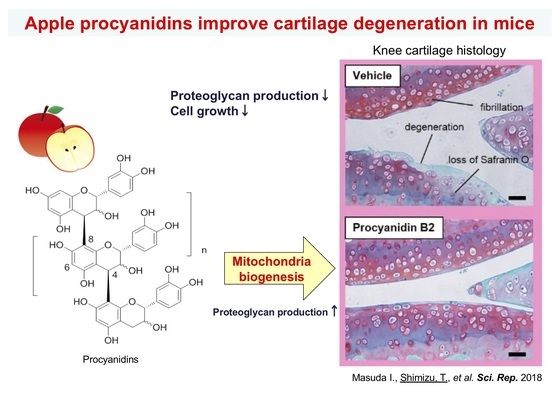
Figure 8. Apple procyanidins improve cartilage degeneration in cartilage-specific Sod2-deficient mice by mitochondrial biogenesis and proteoglycan production.
先頭へ戻る
Members
| Project Leader |
Takahiko Shimizu |
| Research Fellow |
Shuichi Shibuya |
| Kenji Watanabe |
| Research Assistant |
Rina Yamamoto |
| Honoka Sato |
| Visiting Researcher |
Akira Shimamoto |
| Hidetoshi Nojiri |
| Sunao Takeshita |
| Kazuma Murakami |
先頭へ戻る
Publications
2009〜、108 publications (* shows correspondence author)
2021
- Shibuya, S., Watanabe, K., Ozawa, Y., Shimizu, T.*. Xanthine oxidoreductase-mediated superoxide production is not involved in the age-related pathologies of Sod1-deficient mice. Int J. Mol. Sci. 22(7), 3542 (2021).
- Watanabe, K., Shibuya, S., Ozawa, Y., Toda, T., Shimizu, T.*. Pathological relationship between intracellular superoxide metabolism and p53 signaling in mice. Int J. Mol. Sci. 22(7), 3548 (2021).
2020
- Watanabe, N., Noda, Y., Iimura, K., Shimizu, T., Hotta, H.*. Cerebral artery dilation during transient ischemia is impaired by mechanical barrier of amyloid β deposition around cerebral artery in Alzheimer’s disease model mice. J. Physiol. Sci. 70, 57 (2020).
- Sharma, S., Bhattarai, S., Ara, H., Sun G., St. Clair, D.K., Bhuiyan, S., Kevil, C., Watts, M.N., Dominic, P., Shimizu, T., McCarthy, K.J., Sun, H., Panchatcharam, M.*. SOD2 deficiency in cardiomyocytes defines defective mitochondrial bioenergetics as a cause of lethal dilated cardiomyopathy. Redox Biology 37, 101740, (2020).
- Obata,Y., Murakami,K.,* Kawase,T., Hirose,K., Izuo,N., Shimizu,T. and Irie,K.* Detection of Amyloid β Oligomers with RNA Aptamers in AppNL‑G‑F/NL‑G‑F Mice: A Model of Arctic Alzheimer’s Disease. ACS Omega 2020, 5, 21531−21537 (2020).
- Teratani, T., Tomita, K.*, Toma-Fukai, S., Nakamura, Y., Itoh, T., Shimizu, H., Shiraishi, Y., Sugihara, N., Higashiyama, M., Shimizu, T., Inoue, I., Takenaka, Y., Hokari, R., Adachi, T., Shimizu, T., Miura, S. Kanai, T. Redox-dependent PPARg/Tnpo1 complex formation enhances PPARg nuclear localization and signaling. Free Radic. Biol. Med. 156, 45-56 (2020).
- Murakami, K.*, Obata, Y., Sekikawa, A., Ueda, H., Izuo, N., Awano, T., Takabe, K., Shimizu, T., Irie, K.* An RNA aptamer with potent affinity for a toxic dimer of amyloid β42 has potential utility for histochemical studies of Alzheimer’s disease. J. Biol. Chem. 295, 4870-4880 (2020).
- Shibuya, S., Toda, T., Ozawa, Y., Yata, M.J.V., Shimizu, T.* Acai extract transiently upregulates erythropoietin by inducing a renal hypoxic condition in mice. Nutrients, 12(2), 533 (2020).
- Yagi-Yaguchi, Y., Kojima, T., Higa, K., Dogru, M.*, Ibrahim, O., Shimizu, T., Tsubota, K. Shimazaki, J. The effects of 3% diquafosol sodium eye drops on tear functions and ocular surface of the Cu, Zn-superoxide dismutase-1 (Sod1) knock out mice treated with antiglaucoma eye medications. Diagnostics 10, 20 (2020).
- Sagi, H., Shibuya, S., Kato, T., Nakanishi, Y., Tsuboi, A., Moriya, S., Ohno, H., Miyamoto, H., Kodama, H.*, Shimizu, T. SOD1 deficiency alters gastrointestinal microbiota and metabolites in mice. Exp. Gerontol. 130, 110795 (2020).
2019
- Sakamoto, K., Furuichi, Y., Yamamoto, M., Takahashi, M., Akimoto, Y., Ishikawa, T., Shimizu, T., Fujimoto, M., Takada-Watanabe, A., Hayashi, A., Mita, Y., Manabe, Y., Fujii, N., Ishibashi, R., Maezawa, Y., Betsholtz, C., Yokote, K., Takemoto, M*. R3h domain containing-like regulates satellite cell proliferation and differentiation. EMBO Rep., 20, e47957 (2019).
- Kim, J., Funayama, S., Izuo, N., Shimizu, T.* Dietary supplementation of a high-temperature-processed green tea extract attenuates cognitive impairment in PS2 and Tg2576 mice. Biosci. Biotechnol. Biochem. 83(12), 2364-2371 (2019).
- Uchitomi, R., Hatazawa, Y., Senoo, N., Yoshioka, K., Fujita, M., Shimizu, T., Miura, S., Ono, Y., Kamei, Y.* Metabolomic analysis of skeletal muscle in aged mice. Sci. Rep. 9, 10425, DOI: 10.1038/s41598-019-46929-8 (2019).
- Dogru, M.*, Shinzawa, M., Kojima, T., Shimizu, T., Tsubota, K. Age related conjunctival P2Y2 receptor alterations in the Cu, Zn-superoxide dismutase-1 (Sod1)–knockout dry eye model mice. Eye & Contact Lens 45(6), 405-409 (2019).
- Andergassen, D., Muckenhuber, M., Bammer, C.P., Kulinski, M.T., Theussl, H-C., Shimizu, T., Penninger, M.J., Pauler M.F., and Hudson, J.Q.* Deletion of the Airn lncRNA gene shows that RNA mediated silencing of distant imprinted genes does not require any genetic elements within the gene. PLoS Genet. 15(7), e1008268 (2019).
- Shibuya, S., Watanabe, K., Tsuji, G., Ichihashi, M., Shimizu, T.* Platinum and palladium nanoparticles-containing mixture, PAPLAL, does not induce palladium allergy. Exp. Dermatol. 28(9), 1025-1028 (2019).
- Izuo, N., Murakami, K., Fujihara, Y., Maeda, M., Saito, T., Saido, T., Irie, Y., Shimizu, T.* An App knock-in mouse inducing the formation of a toxic conformer of Aβ as a model for evaluating only oligomer-induced cognitive decline in Alzheimer’s disease. Biochem. Biophys. Res. Commun., 515(3), 462-467 (2019).
- Ozawa, Y., Watanabe, K., Toda, T., Shibuya, S., Okumura, N., Okamoto, N., Sato, Y., Kawashima, I., Kawamura, K., Shimizu, T.* Heterosis extends the reproductive ability in aged female mice. Biol Reprod 100(4), 1082-1089 (2019).
- Kim, J., Toda, T., Watanabe, K., Shibuya, S., Ozawa, Y., Izuo, N., Cho, S., Seo, D.B., Yokote, K., Shimizu, T.* Syringaresinol reverses age-related skin atrophy by suppressing FoxO3a-mediated matrix metalloproteinase–2 activation in copper/zinc superoxide dismutase–deficient mice. J. Investig. Dermatol., 139(3), 648-655 (2019).
2018
- Okumura, N., Toda, T., Ozawa, Y., Watanabe, K., Ikuta, T., Tatefuji, T., Hashimoto, K., Shimizu, T.* Royal jelly delays motor functional impairment during aging in genetically heterogeneous male mice. Nutrients, 10(9), E1191 (2018).
- Shimojo, Y., Ozawa, Y., Toda, T., Igami, K., Shimizu, T.* Probiotic Lactobacillus paracasei A221 improves functionality and bioavailability of kaempferol-glucoside in kale by its glucosidase activity. Sci. Rep. 8, 9239, DOI: 10.1038/s41598-018-27532-9 (2018).
- Masuda, I., Koike, M., Nakashima, S., Mizutani, Y., Ozawa, Y., Watanabe, K., Sawada, Y., Sugiyama, H., Sugimoto, A., Nojiri, H., Sashihara, K., Yokote, K., Shimizu, T.* Apple procyanidins promote mitochondrial biogenesis and proteoglycan biosynthesis in chondrocytes. Sci. Rep. 8, 7229, DOI:10.1038/s41598-018-25348-1 (2018).
- Ikeda, K., Simsek, C., Kojima, T., Higa, K., Kawashima, M., Dogru, M.*, Shimizu, T., Tsubota, K., Shimazaki, J. The effects of 3% diquafosol sodium eye drop application on meibomian gland and ocular surface alterations in the Cu, Zn-superoxide dismutase-1 (Sod1)-knockout mice. Graefes Arch. Clin. Exp. Ophthalmol. 256(4), 739-759 (2018)
- Kojima, T., Simsek, C., Igarashi, A., Aoki, K., Higa, K., Shimizu, T., Dogru, M.*, Tsubota, K., Shimazaki, J. The role of 2% rebamipide eye drops related to conjunctival differentiation in the superoxide dismutase-1 (Sod1) knockout mice. Invest. Ophthalmol. Vis. Sci. 59(3), 1675-1681 (2018).
- Morikawa, D., Nojiri, H.*, Itoigawa, Y., Ozawa, Y., Kaneko, K., Shimizu, T. Antioxidant treatment with vitamin C attenuated rotator cuff degeneration caused by oxidative stress in Sod1 deficient mice. J Shoulder Elbow Surg Open Access 2(1), 91-96 (2018).
- Hatazawa, Y., Ono, Y., Hirose, Y., Kanai, S. Fujii, N., Machida, S., Nishino, I., Shimizu, T., Okano, M., Kamei*, Y., Ogawa, Y*. Reduced Dnmt3a increases Gdf5 expression with suppressed satellite cell differentiation and impaired skeletal muscle regeneration. FASEB J 32(3), 1452-1467 (2018).
- Ho, GT.*, Aird, R., Liu, B., Kennedy, N., Dorward, D., Noble, C., Shimizu, T., Morton, N., Rossi, A., Sartor, RB., Iredale, Satsangi, J. MDR1-deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol. 11(1), 120-130 (2018).
2017
- Izuo, N., Kasahara, C., Murakami, K., Kume, T., Maeda, M., Irie, Y., Yokote, K., Shimizu, T.* A Toxic Conformer of Aβ42 with a Turn at 22-23 is a Novel Therapeutic Target for Alzheimer’s Disease. Sci. Rep. 7, 11811, DOI: 10.1038/s41598-017-11671-6 (2017).
- Shibuya, S., Sakaguchi, I., Ito, S., Kato, E., Watanabe, K., Izuo, N., Shimizu, T.* Topical application of trisodiumL-ascorbyl 2-phosphate 6-palmitate 2-phosphate actively supplies ascorbate into skin cells in an ascorbate transporter-independent manner. Nutrients, 9(7), E645 (2017).
- Tuerdi, A. Kinoshita, M., Kamogashira, T., Fujimoto, C. Iwasaki, S., Shimizu, T., Yamasoba, T.* Manganese superoxide dismutase influences the extent of noise-induced hearing loss in mice. Neurosci. Lett. 642, 123-128 (2017).
- Juewon Kim, Si Young Cho, Su Hwan Kim, Donghyun Cho, Sunmi Kim, Chan-Woong Park, Shimizu, T., Jae Youl Cho, Dae Bang Seo*, Song Seok Shin*. Effects of Korean ginseng berry on skin anti-pigmentation and anti-aging via FoxO3a activation. J. Ginseng Res. 41(3), 277-283 (2017).
- Oyama, J.*, Shirakihara, A., Nishikido, T., Maeda, T., Komoda, H., Shimizu, T., Makino, N., Node, K. EGCG, a green tea catechin, attenuates the progression of heart failure induced by the heart/muscle-specific deletion of MnSOD in mice. J Cardiol. 69(2), 417-427 (2017).
2016
- Murakami, K., Tokuda, M., Suzuki, T., Irie, Y., Hanaki, M., Izuo, N., Monobe, Y., Akagi, K., Ishii, R., Tatebe, H., Tokuda, T., Maeda, M., Kume, T., Shimizu, T., Irie, K.*. Monoclonal antibody with conformational specificity for a toxic conformer of amyloid b42 and its application toward the Alzheimer's disease diagnosis. Sci. Rep. 6, 29038 (2016).
2015
- Kojima, T., Dogru, M.*, Ibrahim, O. M., Wakamatsu, T. H., Ito, M., Igarashi, A., Inaba, T., Shimizu, T., Shirasawa, T. Shimazaki, J., Tsubota, K. The effects of 2% rebapimide ophthalmic solution on the tear functions and ocular surface of the superoxide dismutase-1 (Sod1) knockout mice. Invest. Ophthalmol. Vis. Sci. 54(12), 7793-7802 (2015).
- Shibuya, S., Watanabe, K., Yokote, K., and Shimizu, T.* Platinum and palladium nanoparticles regulate the redox balance and protect against age-related skin changes in mice. Textbook of Aging Skin, Springer-Verlag Berlin Heidelberg M.A. Farage et al. (eds.), DOI 10.1007/978-3-642-27814-3_120-1, pp1-11 (2015). ISBN: 978-3-642-27814-3 (Online)
- Koike, M., Nojiri, H., Ozawa, Y., Watanabe, K., Muramatsu, Y., Kaneko, H., Morikawa, D., Kobayashi, K., Saita, Y., Sasho, T., Shirasawa, T., Yokote, K., Kaneko, K., Shimizu, T.* Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 5, 11722 DOI: 10.1038/srep11722 (2015).
- Kobayashi, K., Nojiri, H., Saita, Y., Morikawa, D., Ozawa, Y., Watanabe, K., Koike, M., Asou, Y., Shirasawa, T., Yokote, K., Kaneko, K., Shimizu, T.* Mitochondrial superoxide in osteocyte perturbs canalicular networks in the setting of age-related osteoporosis. Sci. Rep. 5, 9148 DOI: 10.1038/srep09148 (2015).
- Munetomo, A., Hojo, Y., Higo, S., Kato, A., Yoshida, K., Shirasawa, T., Shimizu, T., Kimoto, T., and Kawato, S.* Aging-induced changes in sex-steroidogenic enzymes and sex-steroid receptors in cortex, hypothalamus and cerebellum. J Physiol Sci 65(3), 253-263 (2015).
- Izuo, N., Nojiri, H., Uchiyama, S., Noda, Y., Kawakami, S., Kojima, S., Sasaki, T., Shirasawa, T., Shimizu, T.* Brain-specific superoxide dismutase 2 deficiency causes perinatal death with spongiform encephalopathy in mice. Oxid. Med. Cell. Longev. 2015, 238914, 10 pages (2015).
- Rowley, S., Liang, LP., Fulton, R., Shimizu, T., Day, B., Patel, M.* Mitochondrial respiration deficits driven by reactive oxygen species in experimental temporal lobe epilepsy. Neurobiol. Dis., 75, 151-158 (2015).
- Watanabe, K., Shibuya, S., Ozawa, Y., Izuo, N., Shimizu, T.* Resveratrol derivative-rich melinjo seed extract attenuates skin atrophy in Sod1-deficient mice. Oxid. Med. Cell. Longev. 2015, 391075, 8 pages (2015).
2014
- Shimamoto, A.*, Kagawa, H., Zensho, K., Sera, Y., Kazuki, Y., Osaki, M., Mitsuo Oshimura, Ishigaki, Y., Hamasaki, K., Kodama, Y., Yuasa, S., Fukuda, K., Hirashima, K., Seimiy, H., Koyama, H., Shimizu, T., Takemoto, M., Yokote, K., Goto, M., and Tahara, H.* Reprogramming Suppresses Premature Senescence Phenotypes of Werner Syndrome Cells in Long-Term Culture. PLoS One 9(11), e112900 (2014).
- Sunagawa, T., Masuda, I., and Shimizu, T.* Anti-aging effects of apple procyanidins. Proanthocyanidins: Food Sources, Antioxidant Properties and Health Benefits Chapter 6, pp139-159 (2014). November (Nova Science Publishers).
- Shibuya, S., Ozawa, Y., Watanabe, K., Izuo, N., Yokote, K., and Shimizu, T.* Palladium and platinum nanoparticles attenuate aging-like skin atrophy via antioxidant activity in mice. PLoS One 9(10), e109288 (2014).
- Watanabe, K., Shibuya, S., Ozawa, Y., Nojiri, H., Izuo, N., Yokote, K., and Shimizu, T.* Superoxide dismutase 1 loss disturbs intracellular redox signaling, resulting in global age-related pathological changes. BioMed Res. Int. 2014, 140165, 10 pages (2014).
- Ibrahim, O.M., Dogru*, M., Matsumoto, Y., Igarashi, A., Kojima, Wakamatsu, T.H.F., Inaba, T., Shimizu, T., Shimazaki, J., Tsubota, K. Oxidative stress induced age dependent meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. PLoS One 9(7), e99328 (2014).
- Miyazaki, Y., Kaneko, K., Mizushige, T., Kanamoto, R., Yoshikawa, M., Shimizu, T., and Ohinata, K.* Orally administered a δ opioid agonist peptide rubiscolin-6 stimulates food intake in aged mice with ghrelin resistance. Mol. Nutr. Food Res. 58(10), 2046-2052 (2014).
- Kondo, Y., Masutomi, H., Noda, Y., Ozawa, Y., Takahashi, K., Handa, S., Maruyama, N., Shimizu, T., and Ishigami, A.* Senescence marker protein-30/Superoxide dismutase 1 double knockout mice exhibit increased oxidative stress and hepatic steatosis. FEBS Open Bio. 4, 522-532 (2014).
- Kurokawa, S.*, Niwano, S., Niwano, H., Murakami, M., Ishikawa, S., Masaki, Y., Tamaki, H., Toda, T., Noda, Y., Shimizu, T., Izumi, T., Ako, J. Cardiomyocyte-derived Mitochondrial Superoxide Causes Myocardial Electrical Remodeling by Down-regulating Potassium Channels and Related Molecules.. Cir. J. 78(8), 1950-1959 (2014).
- Murakami, K.*, Irie, K., and Shimizu, T.* Potential role of vitamin C in the prevention of Alzheimer’s disease. Diet and Nutrition Dementia and Cognitive Decline Chapter 61, pp663-668 (2014). (Editor, Preedy, V.R., Elsevier Publishers).
- Kubota, R., Imamura, S., Shimizu, T., Asayama, S., Kawakami, H.* Synthesis of water-soluble dinuclear manganese porphyrin with multiple antioxidative activities. ACS Med. Chem. Letters. 5(6), 639-643 (2014).
- Shibuya, S., Ozawa, Y., Toda, T., Watanabe, K., Tometsuka, C., Ogura, T., Koyama, Y., and Shimizu, T.* Collagen peptides and vitamin C additively attenuate age-related skin atrophy in Sod1-deficient mice. Biosci. Biotechnol. Biochem. 78(7), 1212-1220 (2014).
- Sunagawa, T.*, Shimizu, T., Matsumoto, A., Tagashira, M., Kanda, T., Shirasawa, T., Nakaya, H. Electrophysiological alterations in the heart of heart/muscle-specific Mn-superoxide dismutasedeficient mice. BioMed. Res. Int. 2014, 704291, 12 pages (2014).
- Morikawa, D., Itoigawa, Y., Nojiri, H., Sano, H., Itoi, E., Saijo, Y., Kaneko, K., Shimizu, T.* Contribution of oxidative stress to the degeneration of rotator cuff entheses. J Shoulder Elbow Surg. 23(5), 628-635 (2014).
- Kojima, T., Dogru, M.*, Ibrahim, O. M., Nagata, T., Higa, K., Shimizu, T., Shirasawa, T., Satake, Y., Shimazaki, S. Tsubota, T., Shimazaki, J. The effects of 3% diquafosol sodium application on the tear functions and ocular surface of the Cu,Zn-superoxide dismutase-1 (Sod1)–knockout mice. Mol Vision 20, 929-938 (2014).
- Shibuya, S., Nojiri, H., Morikawa, D., Koyama, H., and Shimizu, T.* Protective effects of vitamin C on age-related bone and skin phenotypes caused by intracellular reactive oxygen species. Aging: Oxidative Stress and Dietary Antioxidants Chapter 14, pp137-144 (2014). (Editor, Preedy, V.R., Elsevier Publishers).
- Shimizu, T.*, Nojiri, H., and Shirasawa, T. Tissue-specifc deletion of Mn-SOD in mice. Systems Biology of Free Radicals and Antioxidants (Editor, Laher, I., Springer Publishers). Chapter 22, 475-487 (2014). Publication Date: April 30, 2014 | ISBN-10: 3642300170 | ISBN-13: 978-3642300172
2013
- Ohguchi, T.*, Kojima, T., Ibrahim, O. M., Nagata, T., Shimizu, T., Shirasawa, T., Kawakita, T., Satake, Y., Tsubota, T., Shimazaki, J., Ishida, S. The effects of 2% rebapimide ophthalmic solution on the tear functions and ocular surface of the superoxide dismutase-1 (Sod1) knockout mice. Invest. Ophthalmol. Vis. Sci. (Investigative Ophthalmology & Visual Science) 54(12), 7793-7802 (2013)
- Kinoshita, M., Sakamoto, T., Kashio, A., Shimizu, T., and Yamasoba, T.* Age-related hearing loss in Mn-SOD heterozygous knockout mice. Oxid. Med. Cell. Longev. 2013, 325702, 13pages (2013).
- Izuo, N., Murakami, K., Sato, M., Iwasaki, M., Izumi, Y., Shimizu, T., Akaike, A., Irie, K.*, and Kume, T.* Non-toxic conformer of amyloid β may suppress amyloid β-induced toxicity in rat primary neurons: implications for a novel therapeutic strategy for Alzheimer’s disease. Biochem. Biophys. Res. Commun. 438(1), 1-5 (2013).
- Watanabe, K., Shibuya, S., Koyama, H., Ozawa, Y., Toda, T., Yokote, K., and Shimizu, T.* Sod1 loss induces intrinsic superoxide accumulation leading to p53-mediated growth arrest and apoptosis. Int. J. Mol. Sci. 14(6), 10998-11010 (2013).
- Morikawa, D., Nojiri, H., Saita, Y., Kobayashi, K., Watanabe, K., Ozawa, Y., Koike, M., Asou, Y., Takaku, T., Kaneko, K., Shimizu, T.* Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. J. Bone Miner. Res. 28(11), 2368-2380 (2013).
- Koyama, H., Nojiri, H., Kawakami, S., Sunagawa, T., Shirasawa, T., Shimizu, T.* Antioxidants improve the phenotypes of dilated cardiomyopathy and muscle fatigue in mitochondorial superoxide dismutase-deficinet mice. Molecules 18, 1383-1393 (2013).
2012
- Kulic, L.*, McAfoose, J., Welt, T., Tackenberg, C., Späni, C., Wirth, F., Finder, V., Konietzko, U., Giese, M., Eckert, A., Kinoshita, N., Shimizu, T., Murakami, K., Irie, K., Rasool, S., Glabe, C., Hock, C., & Nitsch, R. M. Early accumulation of intracellular fibrillar oligomers and late congophilic amyloid angiopathy in mice overexpressing the Osaka intra-Aβ APP mutation. Transl Psychiatry 13(2), e183 (2012).
- Hayakawa, N., Asayama, S., Noda, Y., Shimizu, T., and Kawakami, S.* Pharmaceutical effect of manganese porphyrins on manganese superoxide dismutase deficient mice. Mol Pharm 9, 2956-2959 (2012).
- Sunagawa, T., Watanabe, K., Ozawa, Y., Nakashima, S., Kanda, T., Tagashira, M., Sami, M., Kaneko, T., Tahara, T., Nakaya, H., Shirasawa, T., Shimizu, T.* Apple polyphenols regulate mitochondrial superoxide generation and extend survival in a mouse model of dailated cardiomyopathy. Int J Life Sci Med Res, 2, 46-51 (2012).
- Fujita, H.*, Fujishima, H., Takahashi, K., Sato, T., Shimizu, T., Morii, T., Shimizu, T., Shirasawa, T., Qi, Z., Breyer, M., Harris, C., Yamada, Y. and Takahashi, T.* SOD1, but not SOD3, deficiency accelerates diabetic renal injury in C57BL/6-Ins2Akita diabetic mice. Metabolism 61, 1714-1724 (2012).
- Lustgarten, M., Bhattacharya, A., Muller, F., Jang, Y., Shimizu, T., Shirasawa, T., Richardson, A. and Van Remmen, H.*. Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels. Biochem Biophys Res Commun 422, 515-521 (2012).
- Murakami, K., Murata, N., Noda, Y., Irie, K., Shirasawa, T. & Shimizu, T.* Stimulation of the amyloidogenic pathway by cytoplasmic superoxide radicals in an Alzheimer’s disease mouse model. Biosci Biotechnol Biochem 76, 1098-1103 (2012).
- Takahashi, M.*, Shimizu, T. & Shirasawa, T. Reversal of slow growth and heartbeat through the restoration of mitochondrial function in clk-1-deficient mouse embryos by exogenous administration of coenzyme Q10. Exp Gerontol 47, 425-431 (2012).
- Murakami, K., & Shimizu, T.* Cytoplasmic superoxide radical: a possible contributing factor to intracellular Ab oligomerization in Alzheimer’s disease. Commun. Integ. Biol. 5, 255-258 (2012).
- Kojima, T., Wakamatsu, H. T., Dogru, M.*, Ogawa, Y., Igarashi, A., Ibrahim, O., Inaba, T., Shimizu, T., Noda, S., Obata, H., Nakamura, S., Wakamatsu, A., Shirasawa, T., Shimazaki, J., Negishi, K., Tsubota, K. Age-related dysfunction of the lacrimal gland and oxidative stress: Evidence from the Cu,Zn-superoxide dismutase-1 (Sod1) knockout mice. Am J Pathol 180, 1879-1896 (2012).
- Takahashi, M.*, Ogawara, M., Shimizu, T. & Shirasawa, T. Restoration of the behavioral rates and lifespan in clk-1 mutant nematodes in response to exogenous coenzyme Q10. Exp Gerontol 47, 276-279 (2012).
- Ogawa, K.*, Kim, H., Shimizu, T., Abe, S., Shiga, Y., Calderwood, S. Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress & Chaperones 17, 349-359 (2012).
- Noda, Y., Ota, K., Shirasawa, T. & Shimizu, T.* CuZn-SOD insufficiency impairs progesterone secretion and fertility in female mice. Biol Reprod 86, 1-8 (2012).
- Miura, G., Kato, K., Shimizu, T., Shiga, D., Shirasawa, T.* Heme oxygenase1 is constitutively up-regulated in top climbers. Biochem Biophys Res Commun 417, 104-108 (2012).
- Shibuya, S., Kinoshita, K., Shimizu, T.* Protective effects of vitamin C derivatives on skin atrophy caused by Sod1 deficiency. Handbook of Diet, Nutrition and the Skin Chapter 21, pp350-364 (2012). (Editor, Preedy, V.R., Wageningen Academic Publishers).
2011
- Murakami, K., Murata, N., Noda, Y., Tahara, S., Kaneko, T., Kinoshita, N., Hatsuta, H., Murayama, S., Barnham, K., J., Irie, K., Shirasawa, T. & Shimizu, T.* SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid b oligomerization and memory loss in a mouse model of Alzheimer’s disease. J. Biol. Chem. 286, 44557-44568 (2011).
- Nojiri, H., Saita, Y., Morikawa, D., Kobayashi, K., Tsuda, C., Miyazaki, T., Saito, M., Marumo, K., Yonezawa, I., Kaneko, K., Shirasawa, T., Shimizu, T.* Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J. Bone Miner. Res. 26, 2682-2694 (2011).
- Jones, M. K.*, Zhu, E., Sarino, E. V., Padilla, O. R., Takahashi, T., Shimizu, T., Shirasawa, T. Loss of parietal cell superoxide dismutase leads to gastric oxidative stress and increased injury susceptibility in mice. Am. J. Physiol.-Gastr. L. 301, G537-G546 (2011).
- Toda, T., Sunagawa, T., Kanda, T., Tagashira, M., & Shirasawa, T. Shimizu, T.* Apple procyanidins suppress amyloid-b protein aggregation. Biochem Res Int 2011, 784698 (2011).
- Shimizu, T.*, Baba, T., Ogawara, M., & Shirasawa, T. Lifespan and glucose metabolism in insulin receptor mutant mice. J Aging Res 2011, 315640 (2011).
- Murakami, K., Murata, N., Ozawa, Y., Kinoshita, N., Irie, K., Shirasawa, T. & Shimizu, T.* Vitamin C restores behavioral deficits and amyloid-b oligomerization without affecting plaque formation in a mouse model of Alzheimer's disease. J Alzheimers Dis 26, 7-18 (2011).
- Murakami, K., Yokoyama, S.I., Murata, N., Ozawa, Y., Irie, K., Shirasawa, T. & Shimizu, T.* Insulin receptor mutation results in insulin resistance and hyperinsulinemia but does not exacerbate Alzheimer's-like phenotypes in mice. Biochem Biophys Res Commun 409, 34-39 (2011).
- Parajuli, N., Marine, A., Simmons, S., Saba, H., Mitchell, T., Shimizu, T., Shirasawa, T. & MacMillan-Crow, L.* A. Generation and characterization of novel kidney-specific manganese superoxide dismutase knockout mouse. Free Radic Biol Med 51, 406-416 (2011).
- Toda, T., Noda, Y., Ito, G., Maeda, M. & Shimizu, T.* Presenilin-2 mutation causes early amyloid accumulation and memory impairment in a transgenic mouse model of Alzheimer's disease. J Biomed Biotechnol 2011, 617974 (2011).
- Sasaki, T.*, Shimizu, T., Koyama, T., Sakai, M., Uchiyama, S., Kawakami, S., Noda, Y., Shirasawa, T. & Kojima, S. Superoxide dismutase deficiency enhances superoxide levels in brain tissues during oxygenation and hypoxia-reoxygenation. J Neurosci Res 89, 601-610 (2011).
- Murakami, K.*, Shimizu, T. & Irie, K. Formation of the 42-mer amyloid β radical and the therapeutic role of superoxide dismutase in Alzheimer’s disease. J Amino Acid 2011, 654207 (2011). 査読有
- Sunagawa, T., Shimizu, T.*, Kanda, T., Tagashira, M., Sami, M. & Shirasawa, T.* Procyanidins from apples (Malus pumila Mill.) extend the lifespan of Caenorhabditis elegans. Planta Med 77, 122-127 (2011).
- Ogawa, K.*, Seta, R., Shimizu, T., Shinkai, S., Calderwood, S.K., Nakazato, K. & Takahashi, K. Plasma adenosine triphosphate and heat shock protein 72 concentrations after aerobic and eccentric exercise. Exerc Immunol Rev 17, 136-149 (2011).
- Makino, N.*, Maeda, T., Oyama, J.I., Sasaki, M., Higuchi, Y., Mimori, K. & Shimizu, T. Antioxidant therapy attenuates myocardial telomerase activity reduction in superoxide dismutase-deficient mice. J Mol Cell Cardiol 50, 670-677 (2011).
- Lustgarten, M.S., Jang, Y.C., Liu, Y., Qi, W., Qin, Y., Dahia, P.L., Shi, Y., Bhattacharya, A., Muller, F.L., Shimizu, T., Shirasawa, T., Richardson, A. & Van Remmen, H.* MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell 10, 493-505 (2011).
- Shimizu, T.*, Shirasawa, T. Biomarkers for aging. Nihon Yakurigaku Zasshi 138, 60-63 (2011).
- Sakuramoto, H., Uchimaru, J., Naito, H., Waga, T., Sunayama, S., Shimizu, T., Kurosawa, H. & Shirasawa, T.* Dietary factors alter the oxygen affinity of hemoglobin. Juntendo Med J 57, 624-629 (2011).
2010
- Murakami, K., Horikoshi-Sakuraba, Y., Murata, N., Noda, Y., Masuda, Y., Kinoshita, N., Hatsuta, H., Murayama, S., Shirasawa, T., Shimizu, T. & Irie, K.* Monoclonal antibody against the turn of the 42-residue amyloid β-protein at positions 22 and 23. ACS Chem Neurosci 1, 747-756 (2010).
- Shimojo, Y., Kosaka, K., Noda, Y., Shimizu, T.* & Shirasawa, T.* Effect of rosmarinic acid in motor dysfunction and life span in a mouse model of familial amyotrophic lateral sclerosis. J Neurosci Res 88, 896-904 (2010).
- Shimizu, T.*, Nojiri, H., Kawakami, S., Uchiyama, S. & Shirasawa, T. Model mice for tissue-specific deletion of the manganese superoxide dismutase (Mn-SOD) gene. Geriatr Gerontol Int 10, S70-S79 (2010).
- Murata, N., Murakami, K., Ozawa, Y., Kinoshita, N., Irie, K., Shirasawa, T. & Shimizu, T.* Silymarin attenuated the amyloid beta plaque burden and improved behavioral abnormalities in an Alzheimer's disease mouse model. Biosci Biotechnol Biochem 74, 2299-2306 (2010).
- Murakami, K., Masuda, Y., Shirasawa, T., Shimizu, T. & Irie, K.* The turn formation at positions 22 and 23 in the 42-mer amyloid β peptide: The emerging role in the pathogenesis of Alzheimer’s disease. Geriatr Gerontol Int 10, S169-S179 (2010).
- Taneike, M., Yamaguchi, O., Nakai, A., Hikoso, S., Takeda, T., Mizote, I., Oka, T., Tamai, T., Oyabu, J., Murakawa, T., Nishida, K., Shimizu, T., Hori, M., Komuro, I., Shirasawa, T., Mizushima, N. & Otsu, K.* Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6, 600-606 (2010).
- Kuwahara, H., Horie, T., Ishikawa, S., Tsuda, C., Kawakami, S., Noda, Y., Kaneko, T., Tahara, S., Tachibana, T., Okabe, M., Melki, J., Takano, R., Toda, T., Morikawa, D., Nojiri, H., Kurosawa, H., Shirasawa, T. & Shimizu, T.* Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic Biol Med 48, 1252-1262 (2010).
- Kinouchi, T.*, Matsuda, A., Kawakami, S., Shimizu, T., Shirasawa, T. & Fujii, N. Influence of oxidative stress on D-aspartyl endopeptidase activity. Chem Biodivers 7, 1398-1402 (2010).
- Shimizu, T.*, Shirasawa, T. Anti-aging research using Mn-SOD conditional knockout mice. Yakugaku Zasshi 130, 19-24 (2010).
2009
- Murakami, K., Inagaki, J., Saito, M., Ikeda, Y., Tsuda, C., Noda, Y., Kawakami, S., Shirasawa, T. & Shimizu, T. Skin atrophy in cytoplasmic SOD-deficient mice and its complete recovery using a vitamin C derivative. Biochem Biophys Res Commun 382, 457-461 (2009).
- Masuda, Y., Uemura, S., Ohashi, R., Nakanishi, A., Takegoshi, K., Shimizu, T., Shirasawa, T. & Irie, K.* Identification of Physiological and Toxic Conformations in Ab42 Aggregates. Chembiochem 10, 287-295 (2009).
- Lustgarten, M.S., Jang, Y.C., Liu, Y., Muller, F.L., Qi, W., Steinhelper, M., Brooks, S.V., Larkin, L., Shimizu, T., Shirasawa, T., McManus, L.M., Bhattacharya, A., Richardson, A. & Van Remmen, H.* Conditional knockout of Mn-SOD targeted to type IIB skeletal muscle fibers increases oxidative stress and is sufficient to alter aerobic exercise capacity. Am J Physiol Cell Physiol 297, C1520-1532 (2009).
- Kawakami, S., Matsuda, A., Sunagawa, T., Noda, Y., Kaneko, T., Tahara, S., Hiraumi, Y., Adachi, S., Matsui, H., Ando, K., Fujita, T., Maruyama, N., Shirasawa, T. & Shimizu, T.* Antioxidant, EUK-8, prevents murine dilated cardiomyopathy. Cir J 73, 2125-2134 (2009).
- Chiba, T., Kamei, Y.*, Shimizu, T., Shirasawa, T., Katsumata, A., Shiraishi, L., Sugita, S., Ogawa, Y., Miura, S. & Ezaki, O. Overexpression of FOXO1 in skeletal muscle does not alter longevity in mice. Mech Ageing Dev 130, 420-428 (2009).
先頭へ戻る
Recruitment
We are looking for researchers. If you are interested, please feel free to contact us.
先頭へ戻る
Contact

Takahiko SHIMIZU
Aging Stress Response Research Project Team
National Center for Geriatrics and Gerontology
〒474-8511
7-430 Morioka-cho, Obu City, Aichi Prefecture, Japan
Mail:shimizut(at)ncgg.go.jp
TEL:+08-0562-44-5651 (Ext. 7363 or 6101)
先頭へ戻る





 ”NEW!
”NEW!







