
Thank you for visiting the website of the Department of Neuroimmune System. Our department was newly established on April 1, 2025. We are dedicated to elucidating the mechanisms underlying the interaction between the nervous and immune systems, with a particular focus on how aging disrupts this complex crosstalk. Through our research, we aim to deepen our understanding of sensory processing and the pathophysiology of age-related diseases, contributing to new insights in the field of neuroimmunology and geroscience.
March 1, 2026 – Koketsu has been appointed as a member of the organizing committee for the 31st Annual Meeting of the Japanese Society of Physical Therapy (term: until February 1, 2027).
February 23, 2026 – A paper co-authored by Mun (first author: Dr. Naoya Matsunaga, Okayama University) has been accepted for publication in Frontiers in Cell and Developmental Biology.
January 30, 2026 – Tanaka presented at the AMED “Multisensing” Research Area Meeting.
January 12, 2026 – Tanaka participated in the 20th International Symposium on Geroscience and Geriatrics (ISGG) .
.
January 5, 2026 – Shinnosuke Koketsu joined our team as a research fellow.
December 25, 2025 – Mun's review article, "Nerve-Associated Macrophages: Key Regulators of Neuroinflammation and Neural Homeostasis" was published in the January issue of Precision Medicine.
December 4-6, 2025 – Tanaka presented our research at the 47th Japanease Association for the Study of Pain (JAPAN PAIN WEEK) Symposium.
November 20, 2025 – Tanaka presented our research at the 5th Geroscience Research Center Seminar.
November 3-5, 2025 – Tanaka and Mun presented our research at the 98th Annual Meeting of the Japanese Biochemical Society.
October 31, 2025 – Our research article was published in the November issue of Allergies & Rheumatic Diseases.
October 17, 2025 – Tanaka presented our research at the 9th FUJITA ICBS Seminar, Fujita Medical University
October 4, 2025 – Tanaka presented our research at the 8th Sensory Frontier Research Symposium.
October 1, 2025 – Megumi Maruyama joined our team as a research assistant.
September 27, 2025 – Our research article was published in the October issue of Allergies & Rheumatic Diseases.
September 26, 2025 – Tanaka presented at the AMED meeting.
September 13, 2025 – Tanaka presented at The 68th Annual Meeting of the Japanese Society for Neurochemistry Symposium.
August 21, 2025 – Mun presented a poster at the 10th NCGG Summer Research Seminar.
July 30, 2025 – Our research article was published in the August extra issue of Allergies & Rheumatic Diseases.
July 24, 2025 – Participated in the 48th Annual Meeting of the Japan Neuroscience Society.
June 26, 2025 – Tanaka gave a lecture titled Introduction to Medical Research to first-year medical students at Nara Medical University.
June 20, 2025 – Our research article was published in the July issue of BIO Clinica.
June 19, 2025 – Tanaka presented at the 2nd Geroscience Research Center Seminar.
June 14, 2025 – Tanaka and Mun participated in the 4th APOE Research Meeting (The ApoE).
June 9, 2025 – Tanaka gave a poster presentation at the Keystone Symposia.
June 1, 2025 – Aung Ye Mun joined our team as a research fellow.
May 13, 2025 – We are recruiting a part-time research assistant.
April 11, 2025 – Tanaka presented at The 9th NCGG/TMIG – ICAH Symposium.
April 1, 2025 – The Department of Neuroimmunology Systems was established. Tatsuhide Tanaka was appointed as Director, and Yukari Kido joined as a research assistant.
We aim to elucidate the mechanisms underlying various age-related physiological changes and diseases through the lens of neuroimmune interactions. Our research investigates how the breakdown in communication between the nervous and immune systems contributes to sensory decline and the onset of age-associated diseases. By exploring this intricate crosstalk, we seek to uncover novel biological mechanisms of aging and geriatric disorders.
As individuals age, the risk of developing various diseases increases, including a marked rise in the prevalence of chronic pain. Pain frequently accompanies a wide range of diseases, and understanding its underlying mechanisms is essential for developing effective treatments. We believe that identifying the processes involved in pain generation and progression can contribute to improving the quality of life in the elderly. Our research further aims to discover new mechanisms of pain pathogenesis and to develop innovative therapeutic strategies specifically targeting age-related pain.
Chronic pain is a major global health issue, affecting millions of people and causing profound physical, psychological, and social impacts. Current treatments, including nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, often fall short in providing effective relief. As a result, developing innovative therapeutic approaches to alleviate chronic pain and improve patients' quality of life remains a critical challenge in modern medicine.
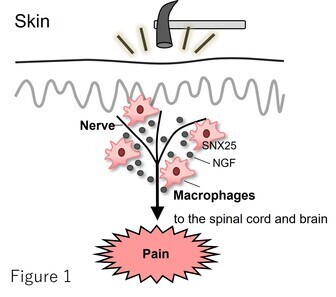
Our previous research has demonstrated that macrophages localized near peripheral nerves within the dermal layer of the skin play a key role in pain modulation through the intracellular trafficking-related factor Sorting Nexin 25 (SNX25). We found that SNX25 promotes the expression and secretion of nerve growth factor (NGF), thereby contributing to the induction of pain (Tanaka et al., Nat. Immunol. 2023, Figure 1).
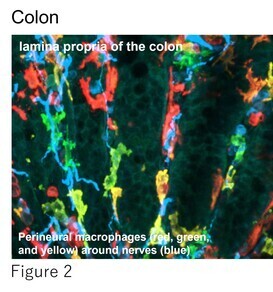
Building on this work, we are now exploring how this sensory regulation system is mediated by nerve-associated macrophages not only in the skin but also in other organs (Figure 2). Our goal is to uncover the underlying molecular mechanisms of pain perception and establish a more comprehensive understanding of pain regulation. Concurrently, we are actively pursuing novel therapeutic strategies targeting SNX25 and its associated factors as potential treatments for chronic pain.
The prevalence of chronic pain increases markedly with age, especially in individuals aged 75 and older. While age-related chronic inflammation is suggested to contribute to chronic pain in the elderly, detailed investigations into the underlying mechanisms remain limited. Our previous studies have demonstrated that alterations in immune cells play a critical role in pain induction. We are now investigating how age-related changes in immune cell function contribute to chronic pain development and persistence.
To elucidate the molecular mechanisms driving chronic pain in the elderly, we employ various pain models using naturally aged mice maintained in our in-house aging colony, “Aging Farm”.
Additionally, we analyze data from the National Institute for Longevity Sciences—Longitudinal Study of Aging (NILS-LSA), a long-term epidemiological study on aging conducted at the National Center for Geriatrics and Gerontology, to examine sensory function and pain in the elderly. This integrative approach allows us to identify age-specific mechanisms and potential therapeutic agents.
Inflammation is a fundamental biological process that defends the host and restores tissue homeostasis by recruiting immune cells to the site of injury. Following peripheral nerve injury, efficient clearance of debris and smooth progression of nerve regeneration are key factors for functional recovery. Among immune cells, macrophages play a central role in the injury response. They contribute to tissue repair and debris clearance through phagocytosis of myelin remnants and secrete either pro- or anti-inflammatory cytokines and growth factors depending on the phase of injury. In addition, macrophages regulate the local immune microenvironment through interactions with other immune cells, such as T and B lymphocytes, and promote axonal regeneration and remyelination in collaboration with Schwann cells.
However, with aging, immune homeostasis becomes dysregulated. Macrophages exhibit reduced phagocytic activity, and immune diversity declines, leading to a persistent, low-grade inflammatory state known as “inflammaging.” These age-associated changes can impair the nerve repair process and contribute to incomplete regeneration.
Our research investigates the cellular and molecular changes in macrophage function using a peripheral nerve regeneration model in aged mice (20–24 months old). These studies aim to lay the foundation for developing innovative regenerative therapies tailored to the needs of the aging population.
Macrophages adhere to the extracellular matrix through specialized structures known as podosomes, which serve as dynamic platforms for sensing external mechanical forces and detecting information such as deformation of the surrounding tissue. We are investigating whether this mechanosensing via podosomes in macrophages is impaired in age-related diseases, aiming to uncover how the interplay between mechanical stress and aging contributes to disease progression. For instance, in conditions such as atherosclerosis and cancer—both of which become more prevalent with age—the affected tissues often become abnormally stiff. Our research seeks to elucidate how macrophages respond to these mechanical alterations through mechanotransduction pathways, ultimately providing novel insights into the pathophysiology of aging and geriatric disorders from a mechanobiological perspective.
Furthermore, we focus on the mechanisms by which mechanical stress is sensed, and we are investigating whether the application of appropriate physical stimuli (mechanical loading) can suppress aging processes and promote the recovery of damaged tissues. We aim to elucidate the molecular mechanisms by which macrophages, upon directly or indirectly sensing physical stimuli, regulate the suppression of inflammation, neuroprotection, and tissue remodeling. Through this work, we seek to establish a novel mechanistic basis for rehabilitation medicine and regenerative therapy.
The defects of the hippocampal ripples and theta rhythm in depression, and the effects of physical exercise on their amelioration. Koketsu S, Matsubara K, Ueki Y, Shinohara Y, Inoue K, Murakami S, Ueki T. Heliyon. 10: e23738. 2023
| Professor | Tatsuhide Tanaka |
|
Researcher
|
Aung Ye Mun Shinnosuke Koketsu |
| Research Assistant |
Yukari Kido Megumi Maruyama |
Matsunaga N, Akiyama K, Mun AY, Zou T, Ito K, Tagashira R, Kuboki T. Prolonged TNF-a stimulation induces a PD-1-associated exhaustion-like phenotype in mesenchymal stroma cells. Frontiers in Cell and Developmental Biology in press
Koketsu S, Matsubara K, Ueki Y, Shinohara Y, Inoue K, Murakami S, Ueki T. The defects of the hippocampal ripples and theta rhythm in depression, and the effects of physical exercise on their amelioration. Heliyon. 10: e23738. 2023
Lab Environment
During this fiscal year, our research environment has been significantly improved through the transfer of equipment as well as the introduction of new and pre-owned instruments. We sincerely appreciate the generous support of everyone involved, and we will continue to advance our research by making full use of these enhanced facilities.

Mun preparing PCR samples

Shinnosuke performing cell treatment

Megumi working under the microscope

Mun observing samples under the microscope

Shinnosuke measuring nucleic acid concentration

Lab meeting
 View from the laboratory
View from the laboratory

November 3-5, 2025 –Mun and Tanaka presented our research at the 98th Annual Meeting of the Japanese Biochemical Society.
August 21, 2025 – Mun presented a poster at the 10th NCGG Summer Research Seminar.
Department of Neuroimmune System,
Geroscience Research Center,
Research Institute,
National Center for Geriatrics and Gerontology
7-430 Morioka-cho, Obu city, Aichi 474-8511, Japan
ttanaka[at]ncgg.go.jp